Why GMOS Are Prohibited in Organic Agriculture?
Author: André Leu
GMO protagonists promote the image that they are only speeding up the natural
crossbreeding used by farmers for millennia by inserting the new gene with the
desired trait directly into the new organisms. They further assert that there is no
evidence of any adverse health effect from the consumption of GMO foods as they
are substantially equivalent to non-GMO foods. This distortion of the facts needs to
be corrected.
The Natural Breeding Misconception
One critical issue is that multiple genes are being transferred across kingdoms and
species such as bacteria, viruses, plants and animals in ways that do not occur by
natural breeding methods.
All living things are classified according to a ranking system that starts with species.
Closely related species are grouped together under a rank that is called a Genus.
Closely related Genera (the plural of genus) are grouped together under the rank of
Family. Closely related Families are grouped together under the rank of Order. There
are seven ranks. Starting with the highest they are: kingdom, phylum or division,
class, order, family, genus, species.
Plants, Animals, Fungi, Viruses and Bacteria belong to separate Kingdoms. Natural
breeding can take place between some species that belong to the same genus and
very occasionally between species of different genera. However species that belong
to different families do not breed and definitely species that belong to different
Kingdoms such as plants, animals, fungi, bacteria and viruses do not breed in nature.
Plants for example do not breed with animals, bacteria or viruses.
Genetic engineering allows for the transfer of multiple genes between Kingdoms in a
way that can never occur naturally. This is something that has never occurred before
and it creates a new frontier with many uncertainties due to science’s limited
understanding about genetics.
The Single Gene Misconception
The other great misconception is that researchers are only inserting one new gene.
At this stage science is not sophisticated enough to insert a single gene and get it to
work. To overcome this problem, scientists have to combine the gene with the
desired trait (such as herbicide tolerance or pesticide production) with other genes
that will make it work (promoter genes). Researchers also insert genes that help
them to identify if the new gene is working within the chromosome (marker genes).
This becomes a complex construction of transgenes, known as a gene cassette, that
can come from bacterial, viral, fish, plant and other sources.
Inserting the Gene Sequence
Another misconception is that the gene is neatly inserted into the cell. Genes are
grouped together inside the cell in long strands call chromosomes. Researchers use
what can be best described as a shotgun approach when they push new genes into a
chromosome. They either shoot the genetic material into the target cells, insert it
after weakening the cell membrane with an electric shock/chemical, or use a
modified microorganism such as a virus or bacteria to infect the target cell with the
new genes. The problem with these approaches is that the researchers do not know
if genes have been inserted into a chromosome, if there are multiple copies, if they
will work and how they will work.
Antibiotic Resistance Marker Genes
The most common method of discovering if the new gene will work involves using
Antibiotic Resistance Marker Genes. In the current generation of commercially
released GMOs, these genes come from bacteria that are resistant to antibiotics. The
marker genes are attached to the gene with the desired trait (herbicide resistance as
an example) and they are shot or infected into the target cells. These cells are then
cultured and an antibiotic is added. The cells that live have adopted the new genes
as they are resistant to the antibiotic.
These are then grown out as plants. The problem with these plants is that every part
of the plant has genes for antibiotic resistance. Many scientists and medical
professionals have expressed concerns about these genes being horizontally
transferred into the gut and mouth bacteria of humans and animals eating genetically
modified food. They are worried that this could create bacteria that are resistant to
the antibiotics needed to cure infections.
Horizontal gene transfer is where microorganisms take up genes directly through
their cell walls rather than by the normal method of reproduction. It has been shown
to occur with the antibiotic resistant super bugs. (Shoemaker et al. 2002)
When the potential danger of this was pointed out to the genetic scientists they
dismissed it as impossible. Several studies have since shown that these antibiotic
resistance genes can be transferred to bacteria in as little as two hours after eating
genetically modified food.
New Scientist in July 2002 reported on a scientific experiment that showed that this
can happen to bacteria in the human digestive system: For the first time, it has been
proved that bacteria in the human gut can take up DNA from genetically modified
food. (Coghlan 2002)
Currently every commercially released GMO plant has the antibiotic resistance genes
in every cell. They should be withdrawn from commercial production for this reason
alone, given the rise in antibiotic resistant human pathogens.
The Cauliflower Mosaic Virus Promoter (CaMV 35S)
When foreign DNA is inserted into organisms, three things usually happen. The most
common one is that the foreign DNA is digested to provide energy and building
blocks for the cell. It can also be rejected. The other response is to close over the
foreign DNA and deactivate it.
All of these responses are defence mechanisms to overcome attacks by pathogens
(disease). The host organism defends itself by getting rid of the foreign proteins. This
is the reason why transplant recipients have to take anti-rejection drugs. When
organisms detect foreign DNA a whole range of responses, collectively known as the
immune system, can be activated to repel or destroy the invaders.
When foreign genes are shot/infected into a cell, they tend to be digested, rejected or
closed over. Either way this means that the target organism will not have the desired
trait from the new gene.
To overcome this, genetic scientists build a construction, called a gene cassette, with
a section of the cauliflower mosaic virus that is called CaMV 35S promoter. The
CaMV gives the signal that activates or promotes the new gene. It ensures that the
gene is active so that its desired trait, like herbicide resistance, works in the new
plant.
Problems with the CaMV 35S
There are several problems with the CaMV. Every current GMO plant is part virus.
Every cell of their bodies contains the active section of a virus. With billions of these
plants now released into the environment, many scientists believe that there is a
great risk of horizontal transfer of the viral genetic code from GMO plants into
invading viruses, creating new virulent transgenic viruses. (Ho et al. 1999, Ho et al.
2009, Ho 2013)
In a paper published in the peer reviewed scientific journal, Microbial Ecology in
Health and Disease, Ho et al. (1999) wrote: ‘It has been known for some time that
recombination can occur between different CaMV viral strains in plants (19), between
different homologous parts of an integrated CaMV viral sequence in transgenic
plants (20) and between an integrated transgene and an infecting virus (21).’
The scientists further stated: ‘It means, in effect, that recombination of the CaMV
promoter elements with dormant, endogenous viruses may create new infectious
viruses in all species to which the transgenic DNA is transferred.’ (Ho et al. 1999)
When GMO scientists and researchers are questioned on this the standard reply is
that the cauliflower mosaic virus is harmless and doesn’t affect humans. Many
harmless viruses change into forms that can be serious. The various forms of the flu
are the classic example. Seventy years ago AIDS and Ebola were restricted to
monkeys and didn’t affect humans. SARS is a slightly modified common cold virus
and is now a seriously fatal disease with the potential for massive epidemics.
According to the journal Nature, April 2003: ‘In a simple overnight experiment,
researchers transformed a coronavirus that is lethal to cats into one that infects
mouse cells by replacing a single gene. The result strengthens the idea that the
SARS coronavirus might have arisen when an animal and human virus met and
swapped genes, says the study’s lead scientist’ (Pearson 2003)
The fact is no scientist can predict what would happen if transgenic viruses and
bacteria emerged from GMO plants. It was only a short time ago these same
scientists were saying pollen drift from GMOs would not affect nearby crops and that
the horizontal transference of antibiotic resistant genes from GMOs into gut
microorganisms was not possible. Ho et al. (1999) concluded that ‘Horizontal transfer
of the CaMV promoter not only contributes to the known instability of transgenic lines
(30), but has the potential to reactivate dormant viruses or creating new viruses in all
species to which it is transferred, particularly in view of the modularity and interchangeability
of promoter elements (8).’
Adverse health effect from GMOs
The GMO industry states that there is no evidence that the consumption of GMOs
has resulted in any adverse health effects in animals or humans. They state that they
are substantially equivalent to non-GMOs so that any concerns are considered as
irrational emotional concerns that are not validated by science. The industry literature
is a classic case of cherry picking data by ignoring a substantial body of evidence
showing a range of adverse reaction to GMOs in published studies.
Zdziarski et al. 2014 conducted a meta review of all the long-term published
comparison feeding studies of GM produce that contains the three most common
specific traits found in commercialized GM crops: herbicide tolerance via the EPSPS
gene and insect resistance via cry1Ab or cry3Bb1 genes.
The researchers only found 21 studies for nine (19%) out of the 47 crops approved
for human and/or animal consumption. They could find no relevant peer reviewed
studies on the other 38 (81%) approved crops. They further found that most of the
studies (76%) were performed after the crop had been approved for human and/or
animal consumption, with half of these being published at least nine years after
approval, which showed that overwhelming majority of GM crops have been
approved without published peer reviewed feeding and safety studies. Of equal
concern, the researchers found inconsistencies in the methodologies and a lack of
defined criteria for outcomes that would be considered toxicologically or
pathologically significant. Critically, they found a lack of transparency in the methods
and results, which made comparisons between the studies difficult. They stated:
‘The evidence reviewed here demonstrates an incomplete picture regarding the
toxicity (and safety) of GM products consumed by humans and animals.’ (Zdziarski et
al. 2014)
This study clearly shows that GM crops are being approved for animal and human
food despite a lack of published evidence based scientific feeding studies to show
that they are safe. By not requiring these studies to generate evidence of safety,
regulators are in effect approving GM crops on the basis of data free assumptions.
Seralini et al. (2011) published a study that reviewed 19 studies of animals fed with
GMO soy and corn in the peer reviewed scientific journal Environmental Sciences
Europe. The studies covered more that 80% of the GMO varieties that are widely
cultivated around the world.
Their review found significant levels of negative effects to kidneys and livers in the
animals that were fed GMOs. The scientists stated: ‘…the kidneys were particularly
affected, concentrating 43.5% of all disrupted parameters in males, whereas the liver
was more specifically disrupted in females (30.8% of all disrupted parameters).’
(Seralini et al. 2011)
One of the key conclusions is that the current testing methodologies (small sample
sizes, inadequate doses, inappropriate controls), length of feed trials (only 90 days)
and the parameters measured (lack of toxicological testing and data) are insufficient
to evaluate the health problems that are caused by diets of GMOs. The scientists
clearly stated that this lack of proper testing protocols is socially unacceptable in
terms of consumer health protection.
Substantially non-equivalent
Regulatory authorities use a basic chemical analysis of a GM variety and compare
this with it closest non-GM variety. If this chemical composition is much the same, the
GM variety is declared as substantially equivalent and suitable for uncontained
commercial release.
Bohn et al. (2014) were able to discriminate between organic, conventional and GE
soybeans without exception, based on vitamin, fat and protein content. Furthermore,
they were able to distinguish GM soybeans from both conventional and organic by
their glyphosate and AMPA (glyphosate degradation product) residues, as well as
substantial non-equivalence in numerous compositional characteristics of soybeans.
The researchers stated, “Using 35 different nutritional and elemental variables to
characterise each soy sample, we were able to discriminate GM, conventional and
organic soybeans without exception, demonstrating ‘substantial non-equivalence’ in
compositional characteristics for ‘ready-to-market’ soybeans” (p. 207).
Multiple Health Problems
A peer-reviewed study published by Swanson et al. (2014) found a strong correlation
between the rise in the production of GMOs, use of glyphosate and 22 chronic
diseases in the USA such as diabetes, obesity, lipoprotein metabolism disorder,
Alzheimer’s, senile dementia, Parkinson’s, multiple sclerosis and autism.
The study showed how this rise in glyphosate exposure is due to the increase in
glyphosate tolerant GMO crops in the USA and cited numerous scientific studies
showing how glyphosate can cause or increase susceptibility to these diseases.
Seralini et al. (2011) published a study that reviewed 19 studies of animals fed with
GMO soy and corn in the peer reviewed scientific journal Environmental Sciences
Europe. The studies covered more that 80% of the GMO varieties that are widely
cultivated around the world.
Their review found significant levels of negative effects to kidneys and livers in the
animals that were fed GMOs. The scientists stated: ‘…the kidneys were particularly
affected, concentrating 43.5% of all disrupted parameters in males, whereas the liver
was more specifically disrupted in females (30.8% of all disrupted parameters).’
(Seralini et al. 2011)
One of the key conclusions is that the current testing methodologies (small sample
sizes, inadequate doses, inappropriate controls), length of feed trials (only 90 days)
and the parameters measured (lack of toxicological testing and data) are insufficient
to evaluate the health problems that are caused by diets of GMOs. The scientists
clearly stated that this lack of proper testing protocols is socially unacceptable in
terms of consumer health protection.
GM Maize Linked to Cancer, Liver and Kidney Disease
The only lifetime comparison feeding study found that rats fed a diet that contains a
proportion of GM maize or minute residues of Roundup has resulted in significantly
higher increases of tumours, kidney disease, liver damage and other negative health
effects.
The study led by Professor Gilles-Eric Seralini and published in Food and Chemical
Toxicology has found that both the GM maize and Roundup acted as endocrine
disrupters and resulted in the females dying 2 – 3 times more than the control
animals. (Seralini et al. 2014)
The females that were fed either GM maize or non-GM maize with minute roundup
residues developed large mammary tumours almost always more often than and
before the controls. All the non-control females, except for one that had ovarian
cancer, had mammary hypertrophies (enlarged mammary glands) and in some cases
hyperplasia with atypia (nodules in the mammary glands).
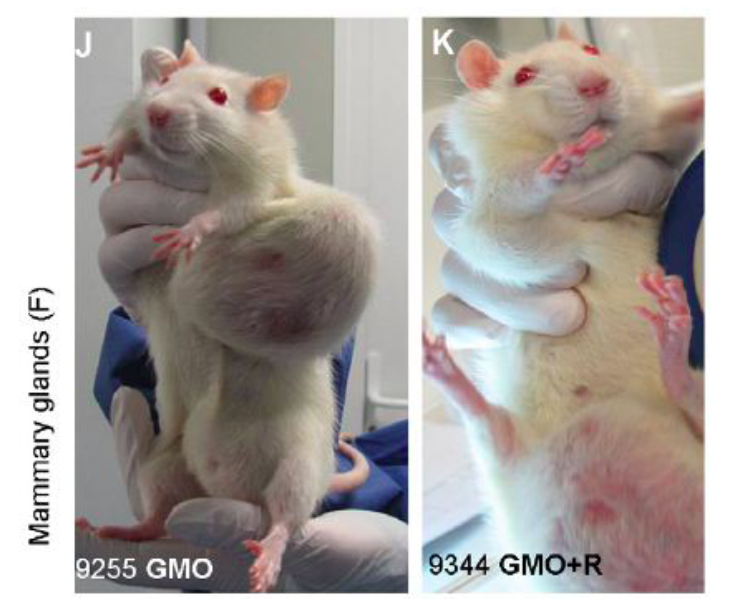
These pictures are examples of the types of mammary gland tumours (breast cancer)
that the scientists found in the rats. Photos permission of Seralini et al 2014.
The pituitary gland was the second most disabled organ and the sex hormonal
balance was modified in females fed with the GMO and Roundup treatments.
The treated males presented 4 times more large palpable tumours (large enough that
they can be felt by hand) than controls and these occurred up to 600 days earlier.
The treated males had liver congestions and necrosis that were 2.5 – 5.5 times higher
than the controls as well as marked and severe kidney nephropathies (kidney
damage) that were also generally 1.3 – 2.3 greater than the controls.
This study was the first 2-year feeding trial conducted on rats designed to see the
effects of a GM diet over an animal’s lifetime. Currently, no regulatory authority
requests mandatory chronic animal feeding studies to be performed for edible GMOs.
However, studies consisting of 90-day rat feeding trials have been conducted by the
biotech industry even though there is no requirement to do this.
The standard comparison of the chemical composition used for establishing
substantial equivalence of the GM maize used in this study (NK603) revealed no
particular difference and consequently it was classified as substantially equivalent.
Similarly, a 90-day feeding trial showed no significant differences between the
controls and the rats fed the GM maize variety.
The results of the 2 year feeding study show that the current regulatory system for
approving GM crops for consumption are inadequate for assessing the effects of
eating these foods over a normal lifetime.
Uterine and stomach damage in pigs
Currently there is only one large sample size published peer reviewed comparison
study of a GM diet in pigs. This is significant as pigs have a gastrointestinal tract and
other organs that are physiologically similar to humans. Carman et al. (2013) found
that the GM diet was associated with gastric and uterine differences in pigs. GM-fed
pigs had uteri that were 25% heavier than non-GM fed pigs. GM-fed pigs had a
higher rate of severe stomach inflammation with a rate of 32% of GM-fed pigs
compared to 12% of non-GM-fed pigs.
Carman et al. (2013) stated: ‘Even though pigs are physiologically similar to humans,
particularly for gastrointestinal observations, very few toxicology studies have been
conducted on them for GM crops (Walsh et al., 2012a). In doing this study, we not
only used animals that were physiologically similar to humans, but we also weighed
and internally examined organs and took blood for biochemical analysis. We further
used a large enough sample size (168 pigs, 84 per group) to be able to determine
statistical significance for key toxicological outcomes.’
GM Soy has adverse effect on the offspring
One of the most concerning issues is the negative effect that occurs in the offspring
of rats and mice that are fed GM diets. These effects include increased infant
mortality, reduced litter sizes and reduced body weights of the offspring.
In experimental trials male and female mice were fed GM soy and then mated. The
early stage embryos (4-8 cells) showed a temporary decrease in gene expression.
This was not found in embryos whose parents ate natural non-GM soy. (Oliveri 2006)
There is strong body of science that shows that subtle changes to gene expression in
embryos can cause permanent negative effects in the development of offspring.
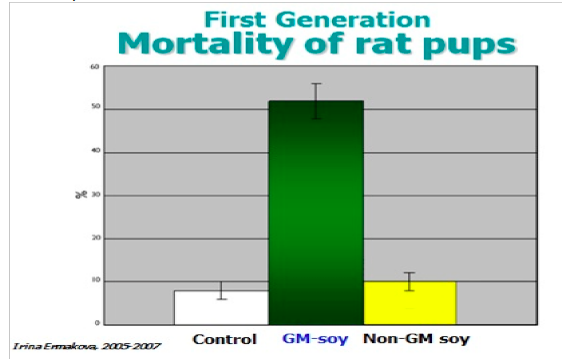
A Russian rat study conducted by Dr Irina Ermakova and colleagues found that
offspring of rats fed in GM soy had higher levels of mortality than rates fed with non-
GMO soy. (Ermakova 2006)
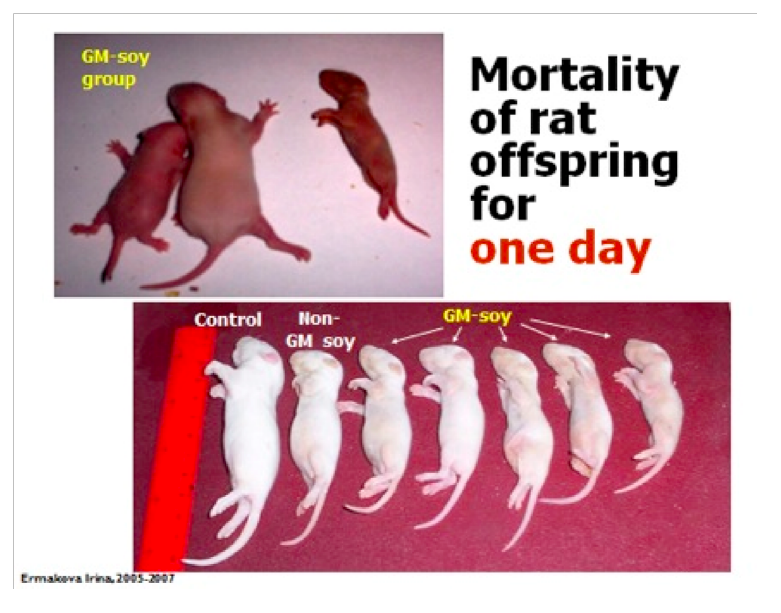
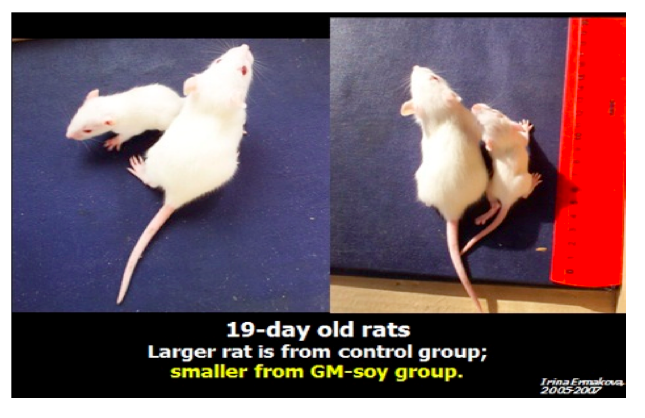
The scientists noted that babies of the rats that were fed GMO diets developed at
slower rate, had lower weights and looked markedly different than the babies of rates
that were fed non-GMO diets. Images permission of Jeffery M Smith

The photo on the upper right is the offspring from mothers fed natural soy. In the
lower left is the GM group. Images permission of Jeffery M Smith
The GMO group has a significant reduction in average weight. Images permission of
Jeffery M Smith
Difficulties with conception
Dr. Ermakova and her colleagues found that the mated offspring of the GM group did
not conceive. This is a serious concern that needs to be fully investigated with more
scientific research. (Ermakova 2006)
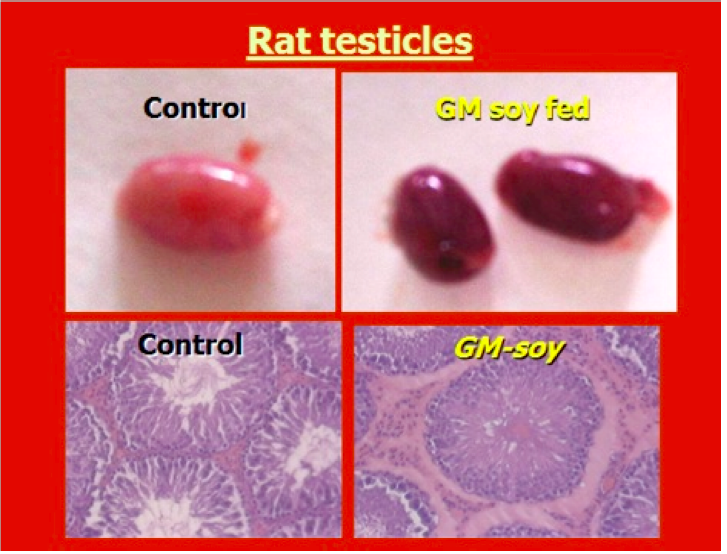
GM soy damages testicles
One of the possible causes for the developmental differences and the lack of fertility
in the offspring of mice that are fed GMOs is that several studies have found that
GMO diets cause structural changes to the testicles. A study published in the
European Journal of Histochemistry found that testicles of mice fed GM soy had
altered structures and functions that influenced sperm development. (Vecchio 2004)
Images permission of Jeffery M Smith
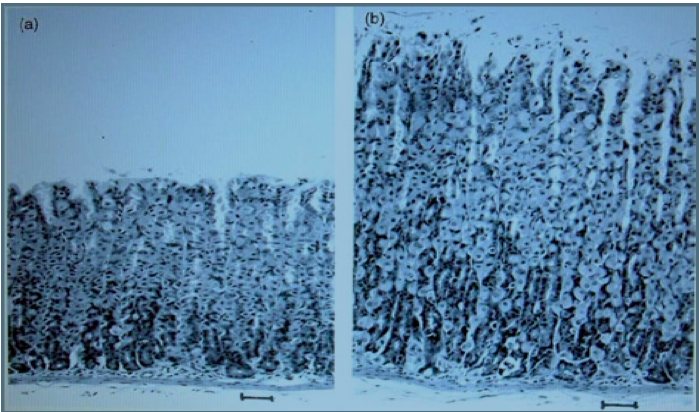
GM potatoes damaged rats
Studies published in highly respected medical journal The Lancet and in the peer
reviewed Journal Nutrition and Health by Dr Arpad Pusztai showed multiple serious
problems with rats that were fed GM potatoes. The scientific studies found that the
rats that were fed on the GMO developed smaller brains, livers and testicles, had
partial atrophy of the liver and damage to immune system. The studies showed that
the rats developed potentially precancerous cell growth in the linings of their stomach
and intestinal walls. (Pusztai 2002, Ewen and Pusztai 1999)
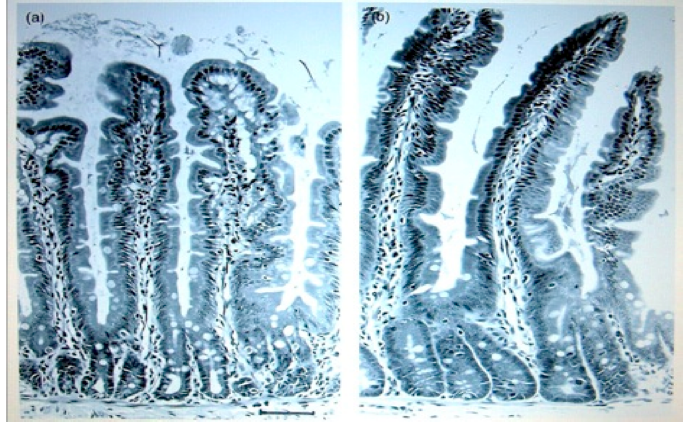
The picture on the left is the intestinal wall of a rat that was fed on non-GMO potato.
Picture on right is from a rat that was fed on GMO potato. These types inflammatory
growths are potentially precancerous and can lead to bowel cancer, which has
become one of the forms of cancer that is increasing in humans.
Images permission of Jeffery M Smith
The picture on the left is the stomach wall of a rat that was fed non-GMO potato. On
the right is the stomach wall of a rat that was fed the GM-potato. These inflammatory
growths are potentially precancerous. Images permission of Jeffery M Smith
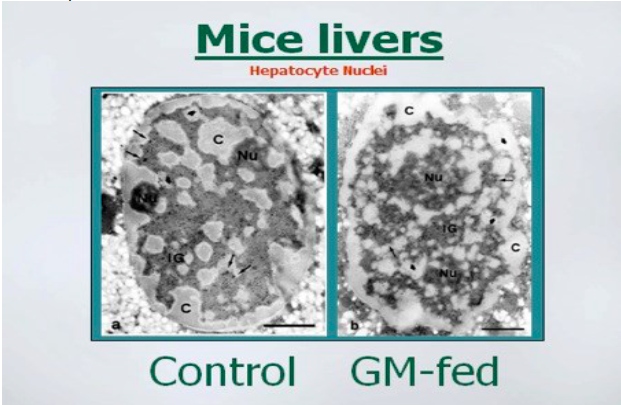
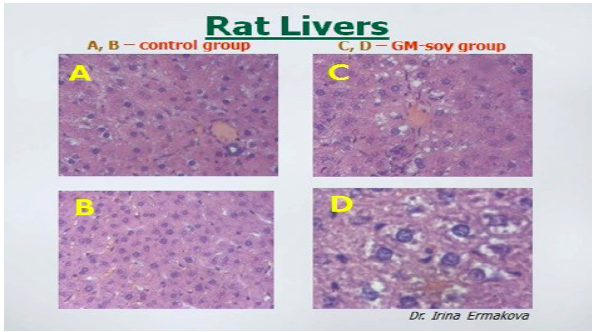
GMO soybean products
There are several animal studies that show range of adverse effects from consuming
GMO soybean products. Mice fed GMO soy for 8 months had a profound drop in the
amount of digestive enzymes produced by their pancreas. (Malatesta 2002 a,
Malatesta 2003). Researcher also found that the liver cells were damaged or
misshapen and there was altered gene expression. They found that there was a
higher rated metabolic activity that suggested that the liver was reacting to a toxic
insult. (Malatesta 2002 b)
The above photos show how the membrane surrounding the nuclei of liver cells was
more irregular in the GM-fed mice. Images permission of Jeffery M Smith.
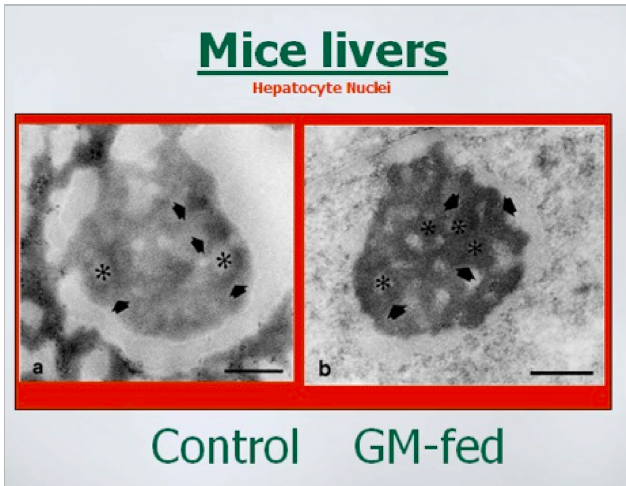
The above photos show that within the nuclei of the liver cells, the structure called the
nucleoli was also misshapen in the GM-fed mice. Images permission of Jeffery M
Smith.
Rats fed GM soy also showed changes in their livers. Images permission of Jeffery M
Smith.
BT Corn
In a study by Monsanto made public because of a lawsuit, rats fed Bt corn developed
signs of liver and kidney toxicity. These included kidney inflammation and kidney
lesions, and decreased kidney weight. The latter symptom is typically related to blood
pressure problems. They also developed increased basophiles that are related to
allergies. The study showed that they had increased lymphocytes or white blood cells
that are part of the immune system indicating a reaction to infection or possibly
disease. There was a 10% increase in blood sugar and decreased immature red
blood cells by 50%. (Burns 2002, Seralini 2007)
GM Corn
When Liberty Link corn was fed to chickens, twice the number of chickens died. But,
the test conducted by the industry was designed so poorly; even a doubling of the
death rate was not statistically significant. (Leeson 1996)
FlaverSavr Tomato
The first GM crop that was looked at by the US FDA was the FlavrSavr tomato,
engineered to have a longer shelf life. Calgene, its producers, were the only
company to give the United States FDA raw feeding study data. They did a study
with rats but the rats refused to eat the tomato.
They force fed rats the FlavrSavr tomato for 28 days. 7 of 20 rats developed stomach
lesions. Another 7 of 40 died within 2 weeks. In the documents made public,
scientists said that the study doesn’t show “a reasonable certainty of no harm.” The
FDA did not block the introduction of the tomato.
The company had created two lines of the GM tomato, both with the same gene
inserted. One was associated with these high rates of lesions and deaths, the other
was not. The company voluntarily decided to market the one that was not associated
with the rat problems.
This also provides an example of how the same crop inserted with identical genes,
may have very different results. And it provides a good example of what can go
wrong with GMOs. (FDA 1993, Pusztai 2002)
GM pea
In Australia, CSIRO researchers took a gene from a kidney bean that produced
proteins that acted as a pesticide, and inserted it into peas to kill the pea weevil. The
researchers did an allergic-type test on mice that no other GMO food crop developer
had done before.
When they exposed mice to the pesticide proteins from the kidney beans, it caused
no reaction. They expected the same to happen when mice were exposed to the
“same” protein produced by the transgene inside the peas. In fact, the amino acid
sequence was identical in both proteins as the one produced by both the bean and
the pea. But the mice developed an inflammatory response to the protein produced in
the GMO peas. It was an immune type response that was very dangerous,
suggesting that the peas might create a deadly anaphylactic shock or other types of
immune or inflammatory reactions in humans.
To understand why the GMO pea caused the severe allergy problems, the
researchers looked very carefully at the protein structure and found that the sugars
that had attached to it had a slightly changed pattern. They said it was the slightly
changed pattern of the sugars that made the peas harmful.
The problem is that the potentially deadly GM peas had already passed all the allergy
tests that are normally used to get GM foods on the market. The only reason they
were stopped was because the crop developer had chosen to use a mice study that
had never been used on any other GM food crop. This shows that the regulatory
system, as practiced, is a failure, and may be letting deadly allergens on the market.
To the credit of the CSIRO they discontinued bringing the GMO pea to commercial
production. (Prescott 2005)
GM L-tryptophan and human deaths
In the late 1980s an epidemic that killed about 100 Americans and caused another 5-
10,000 to fall sick or become permanently disabled was traced to an amino acid
health supplement called L-tryptophan. L-tryptophan is a common amino acid that is
found in milk products. For many years it was extracted from milk and sold as health
supplement to for aiding sleep and calming mood disorders.
A Japanese company Showa Denko started to produce L-tryptophan from genetically
engineering the bacteria. The epidemic was traced back to the L-tryptophan that was
produced from the genetically engineering the bacteria. (Mayeno and Gleich 1994)
Effect on mothers and children
The greatest concern for humans is that the toxin from pesticide producing GMOs
can be found in bloodstream of women and their unborn children. A Canadian study
published in the scientific journal, Reproductive Toxicology, found the pesticide toxin
from GMO crops in the blood samples of women and their unborn babies.
The GMO toxin was found in 93 percent of maternal blood samples and of greater
concern in 80 percent of fetal blood samples. These women were eating the typical
Canadian diet. (Aris and Leblanc 2011)
A peer-reviewed study published in Journal of Applied Toxicology that researched the
combination of the GMO-produced Bt toxin pesticides and Roundup found that they
altered the normal life cycle of cells in human organs. The researchers concluded: “In
these results, we argue that modified Bt toxins are not inert on nontarget human
cells, and that they can present combined side effects with other residues of
pesticides specific to GM plants.” (Mesnage at al. 2013)
Given the evidence of the damaging changes to the offspring of animals fed a GMO
diet, the Canadian study should be the cause of great concern amongst health
professionals and regulators to ensure that the GMO foods that are currently being
consumed are not doing damage to our future generations.
Conclusion
Zdziarski et al. showed there are no studies showing the safety of overwhelming
majority (81%) the main commercially released GMOs. Furthermore they showed
that the existing studies are inadequate to determine that GMOs are safe for human
and animal feed. The data from the published studies cited in this article show that
the assertion by the GMO industry that there is no evidence of damaging health
effects from the consumption of GMO foods is clearly wrong. The data show that the
current situation where there is no mandatory requirement for feeding studies to be
conducted for GMO’s before their commercial release should be seen as neglect of
good science by regulatory authorities. The policy that GMOs are ‘substantially
equivalent’ to non-GMOs and therefore do not need testing is a data free assumption
given that Bohn et al. were able to discriminate GM, conventional and organic
soybeans without exception, demonstrating ‘substantial non-equivalence’ in
compositional characteristics for ‘ready-to-market’ soybeans and that Séralini et al.
were able to demonstrate considerable adverse health outcomes from GM foods
deemed as substantially equivalent. The results of the 2 year feeding study show that
the current regulatory system for approving GM crops for consumption are
inadequate for assessing the effects of eating these foods over a normal lifetime. It
shows that the voluntary 90-day trials used by industry are inadequate to test for
adverse effects and therefore should be disregarded as evidence of safety. The
results of the Carman et al. study shows the need to test these foods on animals that
have gastrointestinal tracts and other organs that are physiologically similar to
humans.
The current testing methodologies (small sample sizes, inadequate doses,
inappropriate controls), length of feed trials (only 90 days) and the parameters
measured (lack of toxicological and histopathological testing and data) are
insufficient to evaluate the health problems that are caused by diets of GMOs. The
research scientists clearly stated that the lack of proper testing protocols is
unacceptable in terms of human health protection.
Until all the commercially released GMOs are tested on animals with gastrointestinal
tracts and other organs that are physiologically similar to humans and that these
tests evaluate the effects of eating these foods over a lifetime, the GMO industry and
the government regulators have no published peer reviewed scientific evidence that
the consumption of GMO foods are safe for humans and animals. In reality they are
using data free assumptions that these novel foods are safe and given that there is a
substantial body of published studies showing adverse health effects, this should be
seen as totally unacceptable.
References Cited
Aris A and Leblanc S, 2011, Maternal and fetal exposure to pesticides associated to
genetically modified foods in Eastern Townships of Quebec, Canada.
Reproductive Toxicology. 2011 May; 31(4):528-33. Epub 2011 Feb 18.
Burns JM, 2002, 13-Week Dietary Subchronic Comparison Study with MON 863
Corn in Rats Preceded by a 1-Week Baseline Food Consumption
Determination with PMI Certified Rodent Diet #5002, December 17, 2002
Bohn T, Cuhra M, Travail T, Sanden M, Fagan J and Primicerio R, 2014,
Compositional differences in soybeans on the market: glyphosate accumulates
in Roundup Ready GM soybeans. Food Chemistry. 153: 207-15.
Carman JA, Vlieger HR, Ver Steeg LJ, Sheller VE, Robinson GW, Clinch-Jones CA,
Haynes JI, John W and Edwards JW, 2013, A long-term toxicology study on
pigs fed a combined genetically modified (GM) soy and GM maize diet, Journal
of Organic Systems, 8(1): 38-54.
Coghlan A, 2002, GM crop DNA found in human gut bugs, New Scientist, 18 July
2002, https://www.newscientist.com/article/dn2565-gm-crop-dna-found-inhuman-
gut-bugs.html#.VGcUOr7lelI (Last viewed 15 November 2014)
Ermakova IV, 2006, GMO: Life itself intervened into the experiments, Letter
EcosInform N2 (2006): 3-4.
Ewen SWB and Pusztai A, 1999, Effect of diets containing genetically modified
potatoes expressing Galanthus nivalis lectin on rat small intestine, Lancet,
1999 Oct 16; 354 (9187): 1353-4.]
FDA, 1993, Department of Veterinary Medicine, FDA, correspondence June 16,
1993. As quoted in Fred A. Hines, Memo to Dr. Linda Kahl, 1993, “Flavr Savr
Tomato: . . . Pathology Branch’s Evaluation of Rats with Stomach Lesions
From Three Four-Week Oral (Gavage) Toxicity Studies . . . and an Expert
Panel’s Report,” Alliance for Bio-Integrity (June 16, 1993)
Ho MW, Ryan A and Cummins J, 1999, Cauliflower Mosaic Viral Promoter – A Recipe
for Disaster?, Microbial Ecology in Health and Disease 1999; 11: 194–197
Ho MW, Ryan A and Cummins J, 2009, New evidence links CaMV 35S promoter to
HIV transcription. Microbial Ecology in Health and Disease, 21: 172–174.
Ho M, 2013, The New Genetics and Natural versus Artificial Genetic Modification.
Entropy, 15(11),4748-4781. https://www.mdpi.com/1099-4300/15/11/4748
(Viewed: 14 October, 2013)
Leeson S, 1996, The Effect of Glufosinate Resistant Corn on Growth of Male Broiler
Chickens, Department of Animal and Poultry Sciences, University of Guelph,
Report No. A56379, July 12, 1996.]
Malatesta M et al., 2002 a, Ultrastructural Analysis of Pancreatic Acinar Cells from
Mice Fed on Genetically modified Soybean, Journal of Anatomy 201, no. 5
(November 2002): 409;
Malatesta M, Caporaloni C, Gavaudan, Rocchi M.B, Serafini S, Tiberi C, Gazzanelli
G, 2002 b, Ultrastructural Morphometrical and Immunocytochemical Analyses
of Hepatocyte Nuclei from Mice Fed on Genetically Modified Soybean, Cell
Struct Funct. 27 (2002): 173-180
Malatesta M, Biggiogera M, Manuali E, Rocchi M. B. L., Baldelli B, Gazzanelli G,
2003, Fine Structural Analyses of Pancreatic Acinar Cell Nuclei from Mice Fed
on GM Soybean, Eur J Histochem 47 (2003): 385-388.
Mayeno AN and Gleich GJ, 1994, Eosinophilia-myalgia syndrome and tryptophan
production: a cautionary tale. Tibtech 12, 346-352. Morphometrical and
Immunocytochemical Analyses of Hepatocyte Nuclei from Mice Fed on
Genetically Modified Soybean,” Cell Struct Funct. 27 (2002): 173-180
Mesnage R et al., 2013, Cytotoxicity on Human Cells of Cry1Ab and Cry1Ac Bt
Insecticidal Toxins Alone or with a Glyphosate-Based Herbicide, Journal of
Applied Toxicology 33, no. 7 (July 2013): 695–99. Originally published online
February 2012
Oliveri et al., 2006, Temporary Depression of Transcription in Mouse Preimplantation
Embryos from Mice Fed on Genetically Modified Soybean, 48th
Symposium of the Society for Histochemistry, Lake Maggiore (Italy),
September 7-10, 2006.
Pearson H, 2003, Deadly virus effortlessly hops species – Genetic engineering helps
reveal origin of deadly ‘flu, Nature doi:10.1038/news030331-4, Published
online 2 April 2003,https://www.nature.com/news/2003/030331/full/news030331-
4.html (last viewed 15 November 2014)
Prescott VE et al., 2005, Transgenic Expression of Bean r-Amylase Inhibitor in Peas
Results in Altered Structure and Immunogenicity, Journal of Agricultural Food
Chemistry (2005): 53.
Pusztai A, 2002, Can science give us the tools for recognizing possible health risks of
GM food, Nutrition and Health, 2002, Vol 16 Pp 73-84;
Seralini GE, Cellier D and Spiroux de Vendomois J, New analysis of a rat feeding
study with a genetically modified maize reveals signs of hepatorenal toxicity by
(2007) Arch. Environ. Contam. Toxicol. 52, 596-602.
Seralini GE, Clair E, Mesnage R, Gress S, Defarge N, Malatesta M, Hennequin D
and Spiroux de Vendômois J, 2014, Republished study: long-term toxicity of a
Roundup herbicide and a Roundup-tolerant genetically modified maize.
Environmental Sciences Europe, 2014: 14.
Seralini GE, Mesnage R, Clair E, Gress S, de Vendomois J and Cellier D, 2011,
Genetically modified crops safety assessments: present limits and possible
improvements. Environmental Sciences Europe, 23: 10.
Shoemaker NB, Vlamakis H, Hayes K & Salyers AA, 2002, Evidence for extensive
resistance gene transfer among Bacteroides spp. and among Bacteroides and
other genera in the human colon. Applied and Environmental Microbiology
67,561 – 5682001.
Swanson NL, Leu A, Abrahamson J & Wallet B, 2014, Genetically engineered crops,
glyphosate and the deterioration of health in the United States of
America, Journal of Organic Systems, 9(2), 2014
Vecchio L et al, 2004, Ultrastructural Analysis of Testes from Mice Fed on Genetically
Modified Soybean, European Journal of Histochemistry 48, no. 4 (Oct-Dec
2004): 449-454.]
Zdziarski IM, Edwards JW, Carman JA and Haynes JI, 2014, GM crops and the rat
digestive tract: A critical review, Environment International 73 (2014) 423–433